OASIS Matrix products are naturally derived extracellular matrix (ECM) scaffolds that support the body’s ability to repair and rebuild damaged tissue.
Made from porcine small intestinal submucosa (SIS), these matrices closely resemble human dermis in both composition and function.1-13 They provide a biologically active scaffold that promotes cellular migration, angiogenesis, and tissue regeneration.14
Why OASIS Matrix works
Simple to store, proven to perform, OASIS gives you more of what matters for managing chronic and complex wounds.
- Similar in composition to human dermis to support natural healing pathways.1-13
- Helps support the formation of granulation tissue.15-16
- Shown to improve epithelialization and wound closure in chronic leg ulcers and diabetic foot ulcers when used with standard care.17-18
- Sterile, shelf-stable, and versatile.
OASIS Matrix solutions
OASIS Matrix Products are designed to meet your patients where they are. That means across wound types, severity, and care settings. It’s ideal for supporting closure in a variety of wounds and can be used alongside standard care protocols.17-18
The portfolio includes:
OASIS® Wound Matrix
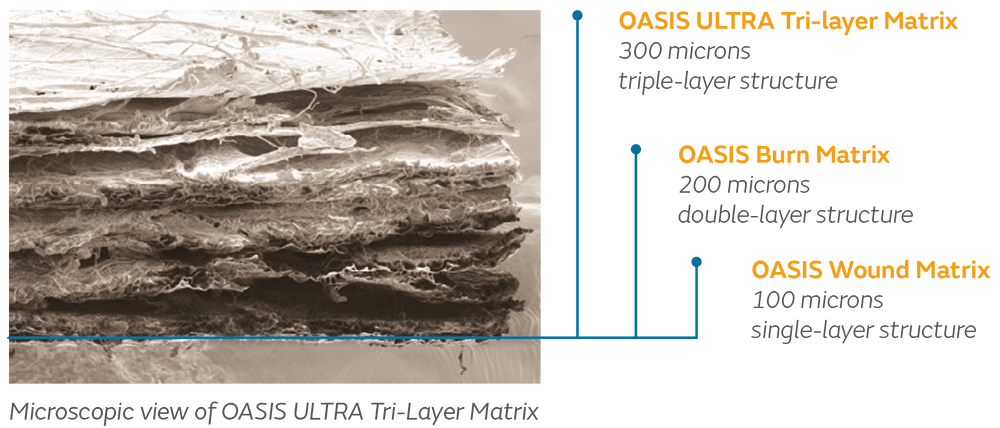
A single layer of fenestrated naturally derived ECM for the shortest timeline to complete integration.
OASIS® Burn Matrix
Two meshed/fenestrated layers of naturally derived ECM. Extra thickness allows for easier fixation and retention of sutures and staples if necessary.
OASIS® Ultra Tri-layer Matrix
Three meshed/fenestrated layers of naturally derived ECM. Additional material allows for weekly visits and challenging wounds with degrading enzymes.
OASIS® XL Matrix
Two-layer structure provides perforated layers of naturally derived ECM for 600cm2 of coverage, ideal for large surface area wounds and burns.
OASIS® MICRO
A micronized powder that the body can breakdown more easily to help kickstart wound edge progression, ideal for tunnelling undermined wounds.
Let’s talk about how OASIS can support better outcomes for your patients and greater efficiency for your team.
- Badylak SF. Semin Cell Dev Biol. 2002;13(5):377-383.
- Frantz C, et al. J Cell Sci. 2010;123(Pt 24):4195-4200.
- Hodde J, et al.. J Mater Sci Mater Med. 2007;18(4):537-543.
- Internal Cook Biotech Document: 97-010 VIIIA.
- Internal Cook Biotech Document: 97-010 VIIIB.
- Internal Cook Biotech Document: 07-057.
- Internal Cook Biotech Document: 00-027.
- Brown B, et al. Tissue Eng. 2006;12(3):519-526.
- Internal Cook Biotech Document: 09-084.
- Hodde JP, et al. Tissue Eng. 1996;2(3):209-217.
- Internal Cook Biotech Document: 96-006.
- Hurst RE, et al. J Biomater Sci Polym Ed. 2001;12(11):1267-1279.
- Sottile J, et al. Mol Biol Cell. 2002;13(10):3546-3559.
- Nihsen ES, et al. Adv Skin Wound Care. 2008;21(10):479-486.
- Cook Biotech, Inc. internal document #04-062.
- Nihsen ES, et al. J Wound Ostomy Continence Nurs. 2007;34(3S):S65.
- Mostow EN, et al. J Vasc Surg. 2005;41(5):837-843.
- Cazzell SM, et al. Adv Wound Care. 2015;4(12):711-718.
- Aboulssa A, et al. Wounds. 2015;27(11):313-318.